

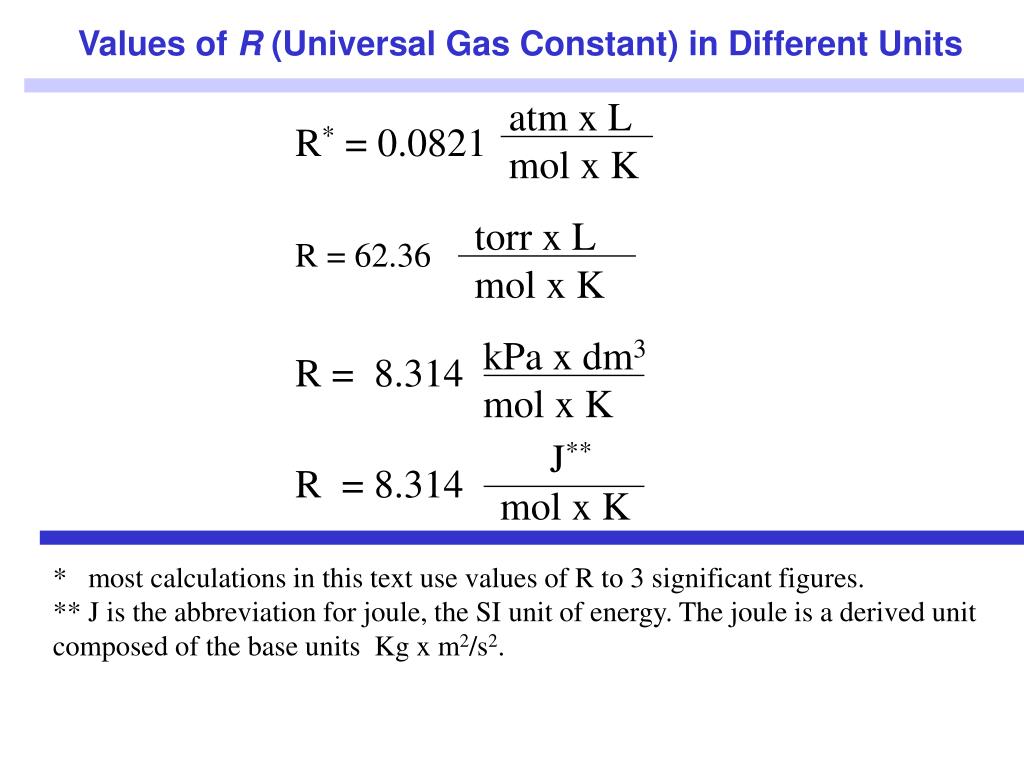
And we will see that in future videos.In chemistry, the formula PV=nRT is the state equation for a hypothetical ideal gas. It's going to be dependent on what units you use for a pressure or volume and temperature. Might be wondering is, "What is this constant?" It's known as the ideal gas constant. We're going to apply it over and over again to Over here, PV is equal to nRT, is one of the most The order right over here, n, which is the number of moles, times some constant times T, our temperature measured in kelvin. This might be looking somewhat familiar to some of you, is equal to, and I'll just change And what will you get? We will get P times V, Or another way to think about it is we can multiply both sides by P. Is just talking about, is gonna be equal to someĬonstant, let's call it R, times all of this business, RnT over P. Or another way to say it is, you could say that volume is going toīe equal to some constant, that's what proportionality The number of moles times the temperature divided by the pressure. Realize that volume is going to be proportional to So we can use these three relationships, and these are actually known as, this first one is known as Boyle's law, this is Charles' law, If you were to take air out, you're also going to decrease the volume, keeping pressure and temperature constant. Temperature constant, you are going to increase the volume.
If you blow air into a balloon, you're putting more Now how does volumeĬompare to number of moles? Well, think about it. Pressure on the outside, you are going to have a lower volume. To exert the same pressure to offset atmospheric And you might say, "Why is it shrinking?" Well, you could imagine that the particles inside the balloon are a little less vigorous at that point. Put it into the fridge, you should see what happens. If you don't believe me, if you take a balloon and you were to blow it up at room temperature, and then if you were to Now how does volume relate to temperature? Well, if I start with my balloon example, and you could run this example Which means that volume would be equal to some constant dividedīy pressure in this case. Pressure is proportional to the inverse of volume. That volume is proportional to one over pressure, So it looks like volume and pressure move inversely with each other. Have inside of the container, it's going to lower the pressure. Make the container bigger, not changing, once again, the temperature or the number of moles I So as volume goes down, pressure goes up. To, per square inch or per square area, exert I have a balloon like this and I have some gas in the balloon, if I try to decrease the volume by making it a smaller balloon without letting out any other air or without changing the temperature, so I'm not changing T and n, what's going to happen to the pressure? Well, that gas is going How does volume relate to pressure? Well, what I imagine is, if So let's think about how these four things can relate to each other. And then we could also think about just how much of that gas we have. And we wanna do it in absolute scale, so we generally measure Although, that pressure would be the same at any point inside of the container. That they would exert on say the inside of the container. We can think about the volume of the container that they are in.

So let's think about how weĬan describe ideal gasses. Some simplifications that approximate a lot of the world. In future videos we'll talkĪbout non-ideal behavior. Two assumptions we make when we talk about ideal gasses. Small fraction of the total volume of the space that they are bouncing around in. But this is a reasonable assumption, because generally speaking, it might be a very, very infinitesimally Now, we know that that isn't exactly true, that individual molecules And we'll also assume that the particles don't Going to study in this video, we'll assume that they don't interact. Light intermolecular forces as they get close to each other or as they pass by each other or if they collide into each other. So in an ideal gas, we imagined that the individual particles Might be wondering is, what is an ideal gas? And it really is a bit ofĪ theoretical construct that helps us describeĪ lot of what's going on in the gas world, or at least close to what's going on in the gas world. Video we're gonna talk about ideal gasses and how we can describe what's going on with them.


 0 kommentar(er)
0 kommentar(er)
